Trihalomethanes and Our Water Supply |
Chlorination has made the U.S. water supply safe from illness producing bacteria, viruses and parasites. Fortunately for our country chlorine disinfection technology has almost completely eliminated from our lives the risks of waterborne diseases such as typhoid fever, cholera, and dysentery. However, the health benefit of chlorination has introduced some possible risks from the byproducts of the disinfection process.
Total Trihalomethanes (TTHM) are a byproduct of chlorinating water that contains natural organics. The water of southwest Florida has always had these organics derived from decaying plant materials and thus, we have probably always had TTHM in our chlorinated water. A U.S. Environmental Protection Agency survey discovered that trihalomethanes are present in virtually all chlorinated water supplies. Many years ago the U.S. Environmental Protection Agency (EPA) required large towns and cities to reduce TTHM levels in potable water. However, recent changes in national drinking water quality standards now require that water treatment systems of smaller towns begin to reduce TTHM. TTHM do not pose a high health risk compared to waterborne diseases, but they are among the most important water quality issues to be addressed in the U.S. water supply.
Comparing risks in our daily lives 
Modern society attempts to reduce risks in our lives starting with the most severe risk factors (hunger, disease, etc.) and continuing to secondary risk factors (traffic laws, cigarette warning labels, etc.). Some people are inclined to exaggerate risks while others completely discount potential problems. We've all heard someone say "If you feed enough of anything to a rat, it will get cancer." While that statement may or may not be true, tests done on laboratory animals are helpful in protecting human health. These test results should not be ignored, nor should they be exaggerated.
The Florida Center for Public Management at FSU ranks drinking water contaminants as a medium to low-level risk. Higher on the scale are indoor air quality, ambient air quality, and food quality. In assessing risks you may also hear comparisons that may or may not be valid, for example "The cancer risk of eating one peanut butter sandwich (containing 2 ppb afiatoxin) is larger than that of drinking a glass of water (containing one ppb chloroform)." That assertion may be comforting for people whose water supplies have one ppb chloroform, but not for people whose water contains ten or more times that amount of chloroform (a component of TTHM).
In the case of TTHM, it does seem prudent to take reasonable steps to reduce this contaminant from our water supply given that some legitimate scientific studies do suggest some health risk and given that the cost of fixing the problem is reasonable. EPA estimates that, nationally, one life might be saved by the investment of $200,000 towards reducing TTHM levels in drinking water. For a given community, however, spending $200,000 on law enforcement, education, health clinics, or some other priority might yield greater life savings than spending that money on TTHM reduction. Thus the dilemma of public health management and governance.
On the individual level, most of us might benefit more by addressing higher risk factors in our lives (driving habits, smoking, exercise, food choices, etc.) before worrying too much about TTHM. For others it may be prudent to take special steps to reduce TTHM. Highest on this list is pregnant women. Pregnant women should discuss this issue with their physicians. While deciding what to do on the household level is an individual decision, deciding what to do on the community level is a governmental decision. After much consideration our federal government has decided that the health questions surrounding TTHM are sufficient to require community water treatment plants take corrective action to achieve low TTHM levels in water.
Some scientific studies have linked TTHM to increased risk of cancer. Several studies suggest a small increase in the risk of bladder cancer and colorectal cancer. Beyond the cancer and reproduction concerns, some investigations have found that chlorination by-products may be linked to heart, lung, kidney, liver, and central nervous system damage. Other studies have linked TTHM to reproductive problems, including miscarriage. A California study found a miscarriage rate of 15.7% for women who drank 5 or more glasses of cold water containing more than 75 ppb TTHM, compared to a miscarriage rate of 9.5% for women with a low TTHM exposure. A North Carolina study investigating the same question but found no strong relationship between TTHM and problem pregnancies. Exposure to TTHMs is not limited only to water you drink. An article in the Washington Post Health Section on (March 12, 2002) stated that one study showed that a 10 minute shower produced more absorbtion of TTHM through the skin than drinking 5 glasses of water. When taken in total, the cancer evidence is probably the strongest among the possible TTHM health risks. For these reasons total trihalomethanes (TTHM) in public water supplies are limited to 0.08 ppm (80 ppb). This represents a reduction in the limit from the previous EPA threshold of 0.1 ppm (100 ppb).
Of the THMM compounds, Dibromochloromethane was the most closely associated with cancer risk, (0.6 ug/l to cause a one in one million cancer risk increase) followed in order by Bromoform, Chloroform, and Dichlorobromomethane. These distinctions among the specific chemical by-products of is a result of toxicological, not empidemiological studies. Current regulations limit the concentration of these four chemicals added together (total trihalomethane or TTHM levels) to 100 ug/l. TTHM can be be found in chlorinated water supplies and in the air of buildings where running water and showers release the chemicals into the room, however, the EPA has determined that this airborne exposure is minimal compared to that from consumption. The National Institutes of Health provides a searchable database on chemical health studies.
ppm = parts per million (1E-6)
mg/L = milligram per liter (same as parts per
million)
ppb = parts per billion (1E-9)
ug/L = micrograms per liter (same as parts per
billion)
One way to reduce TTHM levels is to change from CHLORINE disinfection to CHLORAMINE disinfection. This solution is being employed by both the Port LaBelle Utilities System and the City of LaBelle Public Works. Unlike chlorine, chloramines do not combine with organics in the water to form potentially dangerous trihalomethanes (TTHM). But since chloramines are a somewhat less effective disinfection agent, the amount of disinfectant added must be increased to maintain the proper level of drinking water safety. Therefore a water treatment plant changing from chlorine to chloramine must go through a period of testing to optimize the system performance for the control of TTHM.
Chloramines are formed from the reaction between ammonia and chlorine. Adding ammonia (NH3) to a chlorination system converts the chlorine to chloramine. Chloramines can exist in three forms:
The proportions of the chloramines depend on the physical and chemical properties of the water. Many other communities have converted to this chloramination system. By switching from chlorination to a chloramine process, the tap resulting water may have more color. Color comes from natural organics in the water (tannins, humics) derived from decayed plant matter. Because the chlorine is no longer reacting with and consuming the organics, greater amounts of color may remain in the tap water.
Use of chloramines is not the only option available to reduce the production of TTHM in water treatment plants. Other options include:
Removing chloramines from your water

Note: Water containing chloramines may not be used for aquariums or kidney dialysis.
FISH and AQUARIUMS
A variety of commercial products and options are available for removing ammonia, chlorine, and chloramines from aquarium water.
Ammonia Detox
Filters
Kordon
Activated Carbon
Additives
For more information about chloramines and fish, see the Canadian website from the Ottawa Water Division.
HEALTH and KIDNEY DIALYSIS
A number of options are available for removing chloramines from water prior to use by a kidney dialysis machine. These options include using an activated carbon filter. However before using any treatment for kidney dialysis machine water, consult your physician. He or she will recommend the appropriate type of water treatment for you. Additional information about chloramines and their health effects is available from the Ottawa Water Division website.
Back to TOP
What else you can do about TTHM
Until a chloramine system is installed and operational, citizens can take steps in their homes to reduce TTHM if, after considering the facts, they believe it to be an important factor in their lives.
General information about drinking water quality is available from a variety of Internet sources:
Back to TOP
TTHM chemistry and measurements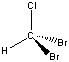
Trihalomethanes are a group of organic chemicals formed in water when chlorine reacts with natural organic matter (such as humic acids from decaying vegetation). Humic acids are present in all natural water used as sources of drinking water. Total trihalomethanes (TTHM) are not a single chemical but a class of compounds that includes:
NATURAL ORGANIC COMPOUNDS IN WATER
- chloroform (CHCl3)
- bromoform (CHBr3)
- dichlorobromomethane (CHCl2Br)
- dibromochloromethane (CHClBr2)
Chlorine reacts with the natural organic carbon compounds in the water to form trihalomethanes. These organic compounds include:
Humic Substances
Humic substances are the organic portion of soil that remains after prolonged microbial decomposition, and that is formed by the decay of leaves, wood, and other vegetable matter. They can impart a yellowish-brown to brownish-black color to water; detectable to 0.1 ppm in water. Humic substances are commonly classified on the basis of solubility. If a material containing humic substances or humus is extracted with a strong base and the resulting solution is then acidified, the products are a) a nonextractable plant residue called humin, b) a material called humic acid that precipitates from the acidified (pH < 2) solution, and c) an organic material called fulvic acid that remains dissolved in the acidified solution. The high molecular weight and polyelectrolytic humic substance macromolecules range from a molecular weight of a few hundred for fulvic acid to tens of thousands for the humic acid and humin fractions. Humic substances are excellent chelating agents that bind with and hold metal ions in water, and they also effectively exchange cations with water.
Fulvic Acid
A water-soluble, natural organic substance of low molecular weight which is derived from humus, often found in surface water. Fulvic acid contributes to the formation of trihalomethanes in chlorinated water supplies, and can contribute to organic fouling of ion exchange resin beds.
Color in water can be caused by a number of contaminants such iron, tannins and humics. Color from iron is referred to as "apparent color" rather than "true color". True color is distinguished from apparent color by filtering the sample. The most common source of true color is decaying organic matter such as the yellowish "tea color" of water. True color is mostly found in surface water, although ground water may contain some color if the aquifer flows through a layer of buried vegetation, such as from a long buried slough of a river. Color is not a toxic characteristic, but is listed by the ADEC as a secondary (aesthetic) parameter affecting the appearance and palatability of the water. Color can be removed by activated carbon filters, sometimes marketed as taste and odor filters. The activated carbon or charcoal must be replaced after a period of time when its capacity for adsorption of the color is exhausted. Another treatment method is coagulation and sedimentation using alum or other chemicals. This process is normally used only in large plants since its complexity requires the care of a trained water treatment plant operator.